The properties of matter are its characteristics that can be observed and measured. These properties describe matter in every possible way. For example, mass describes how much matter we have, volume indicates how much space the matter occupies, flammability tells us whether the matter can catch fire, and the pH of a substance indicates whether it is acidic, basic, or neutral. Together, all of these properties define matter and make it distinguishable from other matters.
However, to better understand these properties, they are classified based on two factors: how they are studied and how they depend on the amount of matter being studied. Based on these two factors, the properties of matter are further sub-divided into four categories.
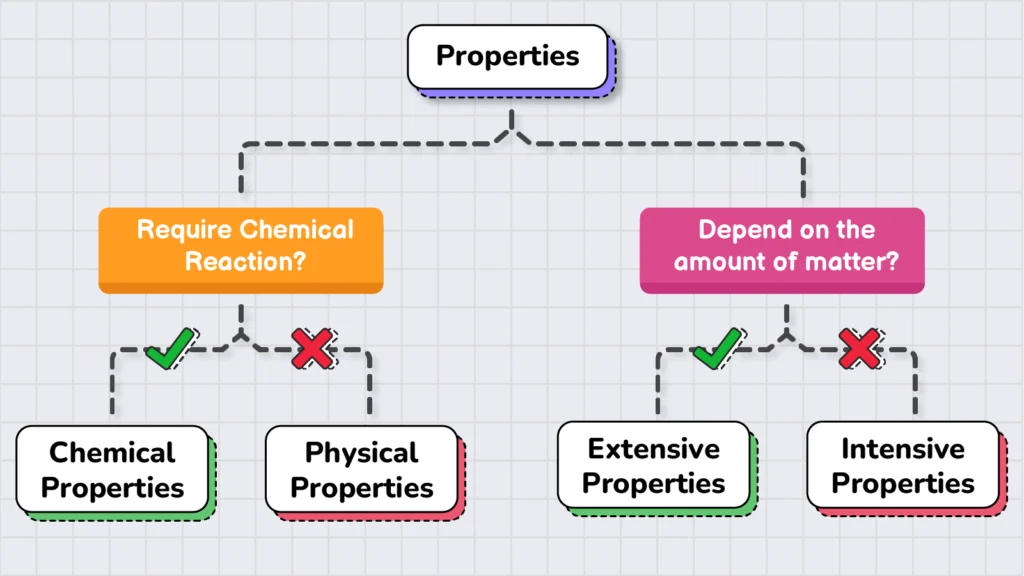
If we categorize properties based on how they are studied, they fall into two broad categories:
- Physical Properties
- Chemical Properties
On the other hand, if we categorize properties based on their relationship to the amount of matter, they are divided into the following two categories:
- Extensive Properties
- Intensive Properties
Classification of Properties based on their study:
Chemical Properties:
The properties of matter that require a chemical reaction to be studied are called chemical properties. A chemical reaction is any process that changes the composition or chemical structure of a substance, or transforms that substance into another substance.
For example, the flammability of a substance is a chemical property. To determine if a substance is flammable, you must burn it, which is a chemical reaction. This burning alters the composition of the substance, converting it into a different substance.
Similarly, corrosivity is also a chemical property. To determine how corrosive a substance is, it must come into contact with another matter. During this interaction, the substance corrodes the other matter and, in the process transforms itself into a different substance.
Physical Properties:
Physical properties of matter are those that do not require a chemical reaction for their study.
For example, mass is a physical property of matter. To measure the mass of any substance, you simply need a balance. After measuring the mass, the substance retains its composition and chemical structure.
Similarly, the boiling point of matter is also a physical property. To study the boiling point of a substance, such as water, you just need to increase the temperature until it boils. During this process, water changes into steam, but both steam and water are essentially different states of the same substance.
Classification of Properties Based on their Relationship to the Amount of Matter:
Extensive Properties:
Properties of matter that depend on the amount of matter being studied are called extensive properties.
Mass and volume are two examples of extensive properties because they directly depend on the quantity of matter. For instance, the glass and jug contain different amounts of water, which means the mass and volume of water in each container will also differ. The mass of the water in the glass is 100 g, with a volume of 100 mL, while the mass of the water in the jug is 400 g, with a volume of 400 mL.
Intensive Properties:
Intensive properties, on the other hand, are independent of the amount of matter being studied; they depend solely on the type of matter.
Boiling point and density are examples of such properties because they remain constant regardless of the amount of matter. Under similar conditions, a given substance will always exhibit specific values for these properties.
For example, water in both a glass and a jug, regardless of the amount, will have the same density (1 g/mL) and boiling point (100°C) under similar conditions.