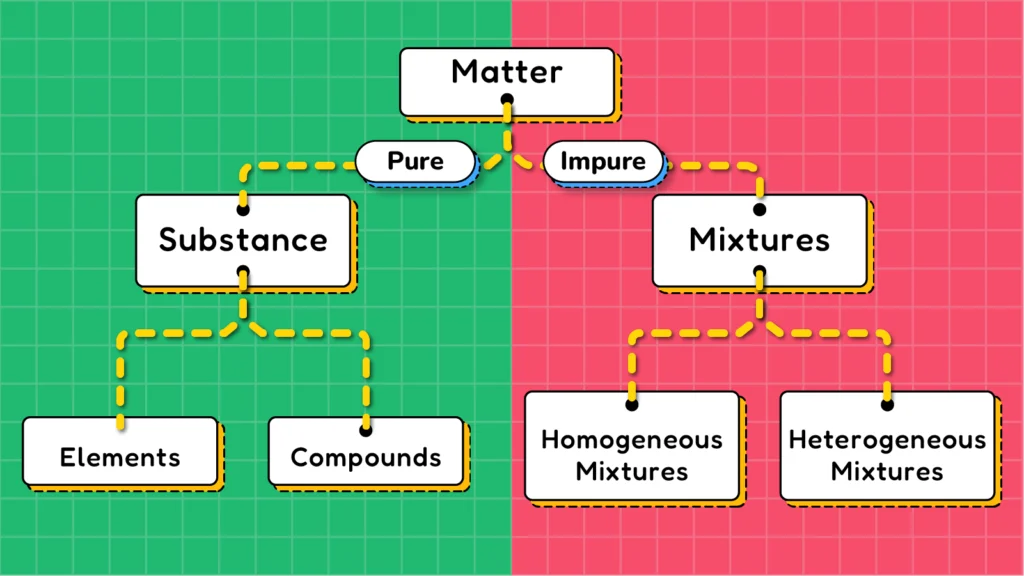
Matter, based on its chemical composition, can be divided into two broad categories: substances and mixtures. These broad categories can then be further divided into subcategories. Substances include elements and compounds, while mixtures are classified as homogeneous mixtures and heterogeneous mixtures.
Understanding these categories is essential for studying chemistry because they provide a framework for classifying and distinguishing different types of matter based on their composition and properties.
Substances:
Substances are considered the purest form of matter, but what does “pure” really mean?
Being pure means that every unit of the substance is identical, sharing the same composition and properties. To better understand this, let’s explore the two main subcategories of substances: elements and compounds.
Elements:
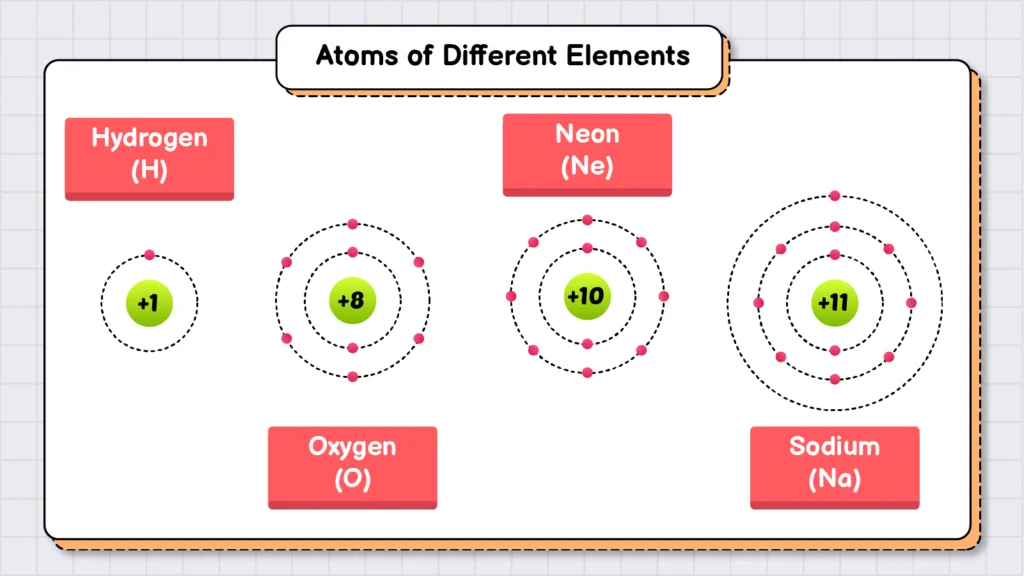
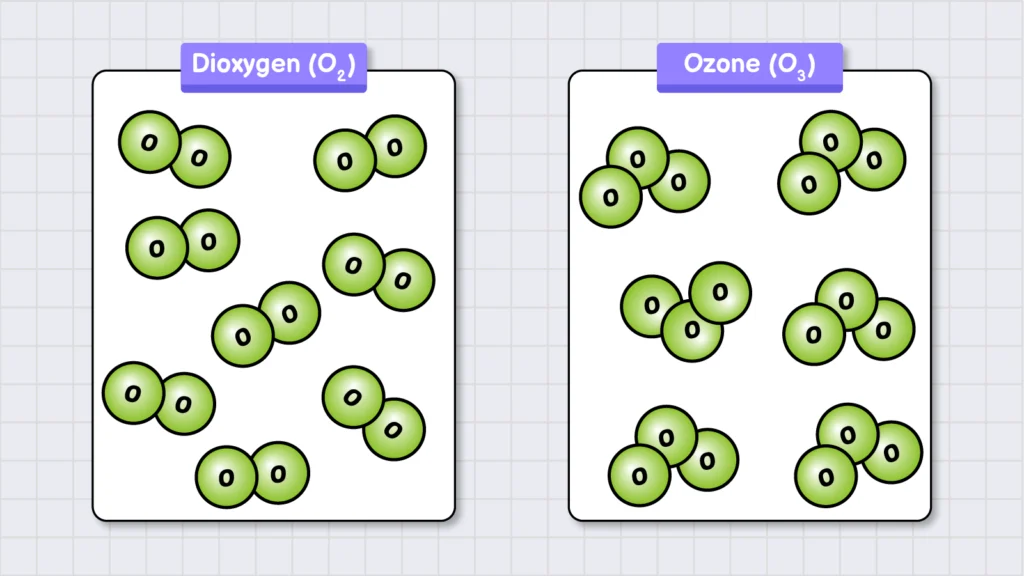
Elements are the simplest and purest form of matter. Every atom of an element has the same number of protons in its nucleus. For example, oxygen is an element. If we take a balloon filled with oxygen gas, it will contain only oxygen atoms, each with 8 protons in their nuclei. However, these oxygen atoms do not exist independently. Instead, they pair up and form O2 molecules. Similarly, ozone (O3) is another form of oxygen element, where three oxygen atoms combine to form a molecule.
It’s also important to note that elements can exist in multiple forms. For example, graphite and diamond are two structurally different forms of the same element, carbon. These forms are called allotropes. Allotropes share some properties while differing in others.
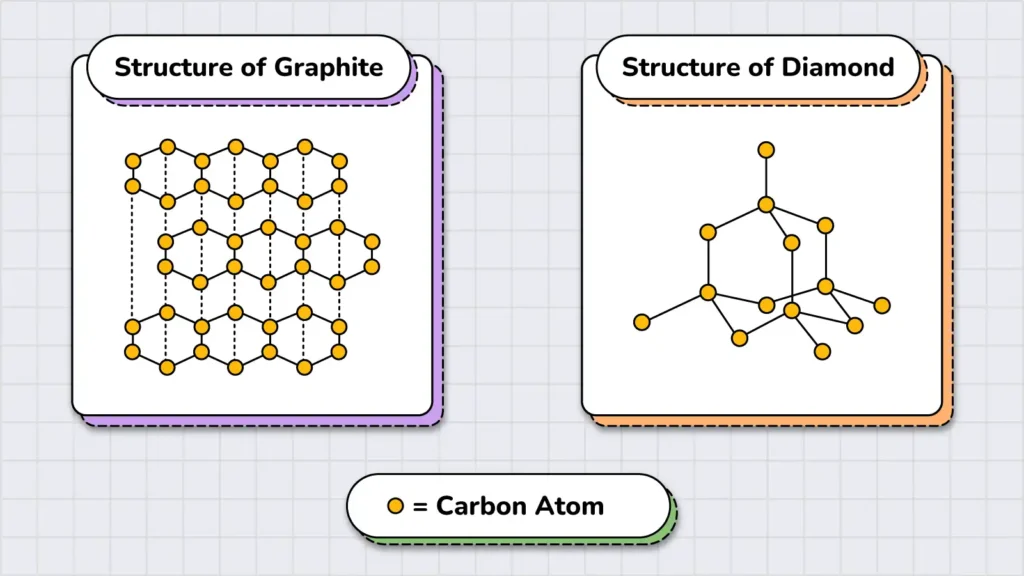
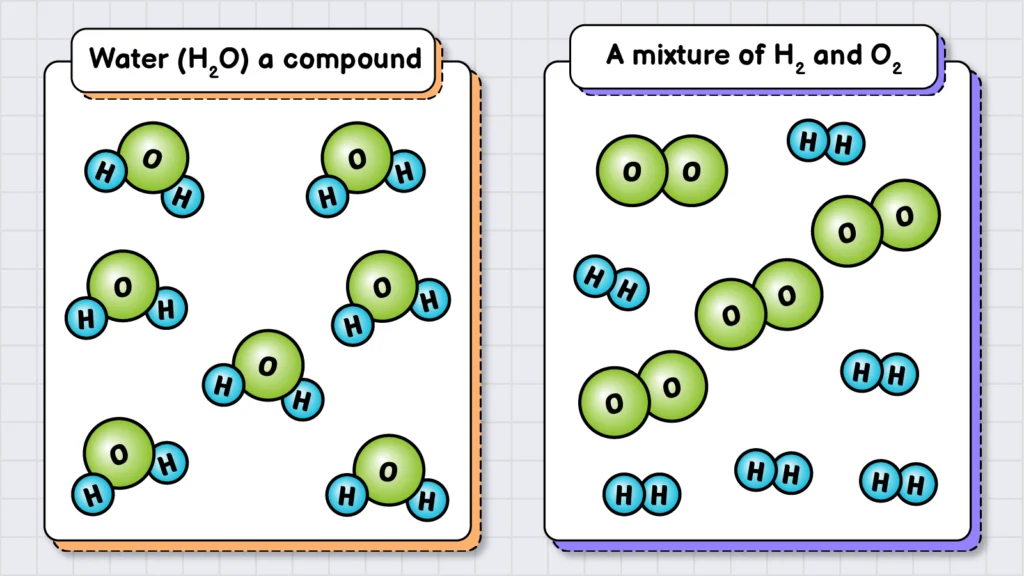
In addition, elements are the fundamental building blocks of all the matter. For instance, when two elements—oxygen (O2) and hydrogen (H2)—are chemically combined in a specific ratio (2:1), they form water (H2O), which is a compound (the second type of substance).
Compounds
Compounds are substances in which each unit is made up of two or more different types of elements chemically combined in a fixed proportion.
In simpler terms, for a substance to be classified as a compound, it must meet three conditions:
- It must contain atoms from at least two different elements.
- The atoms must be chemically bonded, either through ionic or covalent bonds.
- The ratio of the atoms must always be fixed and specific.
For example, water (H2O) is a compound because it fulfills all these conditions. It contains atoms from two elements: hydrogen (H) and oxygen (O). These atoms are chemically bonded, and every unit of water (H2O) has the same fixed ratio of hydrogen to oxygen atoms (2:1). However, if you have a container with both of these elements, but their atoms are not chemically bonded, you cannot call it a compound. Instead, it would be considered a mixture of H2 and O2 gases.
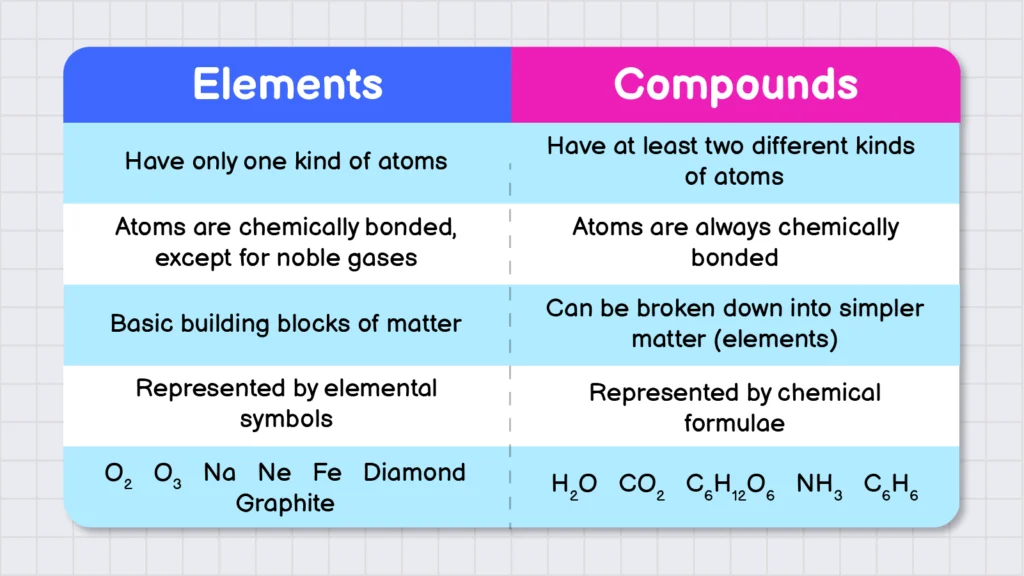
Difference between Elements and Compounds:
Following are some differences between elements and compounds:
Mixtures:
Mixtures are physical combinations of two or more substances, where each substance retains its chemical composition and properties.
In simpler terms, mixtures have the following characteristics:
- They consist of two or more substances.
- The substances are physically mixed, meaning no chemical reaction takes place.
- Each substance retains its own chemical composition and properties.
Based on the composition of mixtures, they can be further divided into two sub-categories: homogenous mixtures and heterogenous mixtures.
Homogeneous Mixtures:
Mixtures with a uniform composition are called homogenous mixtures.
A uniform composition means that the components of the mixture are evenly distributed. For instance, if you dissolve table salt in a glass of water, you create a homogenous mixture because the salt is uniformly distributed throughout the water. In other words, the concentration of salt is uniform throughout the mixture.
Moreover, in homogeneous mixtures, the substances lose their individual identities. For example, air is a mixture of many different substances, but we can’t identify any of them separately. The components are so well-mixed that it’s impossible to distinguish them without the help of specialized instruments.
Heterogenous Mixtures:
In contrast to homogenous mixtures, heterogenous mixtures do not have a uniform composition.
For example, pizza is a heterogeneous mixture because its composition is not uniform. Some parts of the pizza might have more pepperoni, while others might have fewer olives.
Other examples of heterogeneous mixtures include ice cream, salad, cookies, and concrete.
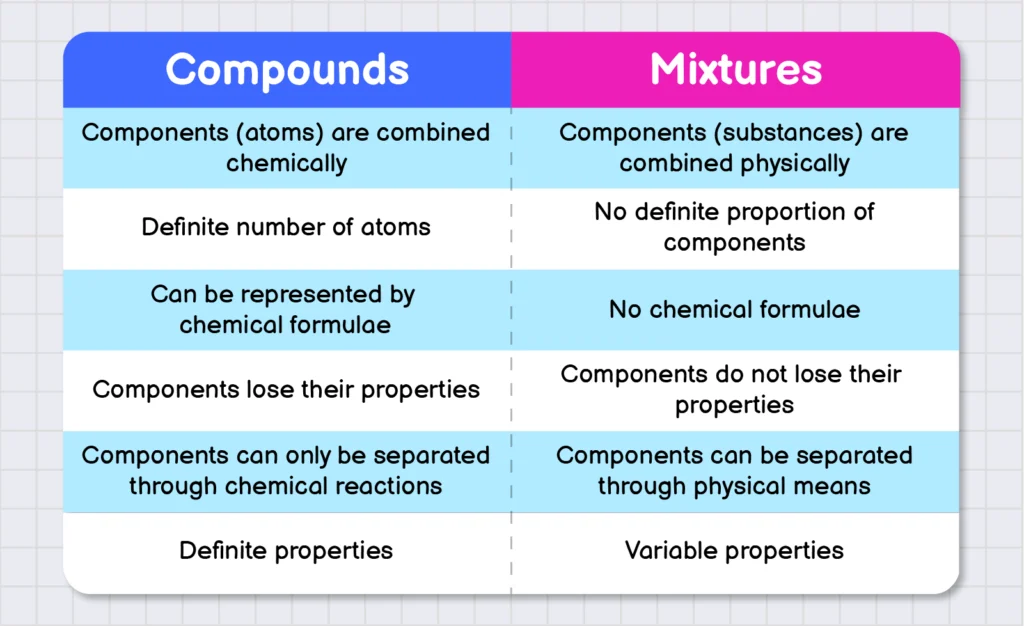
Difference of Compounds and Mixtures:
Following are some differences between elements and compounds: