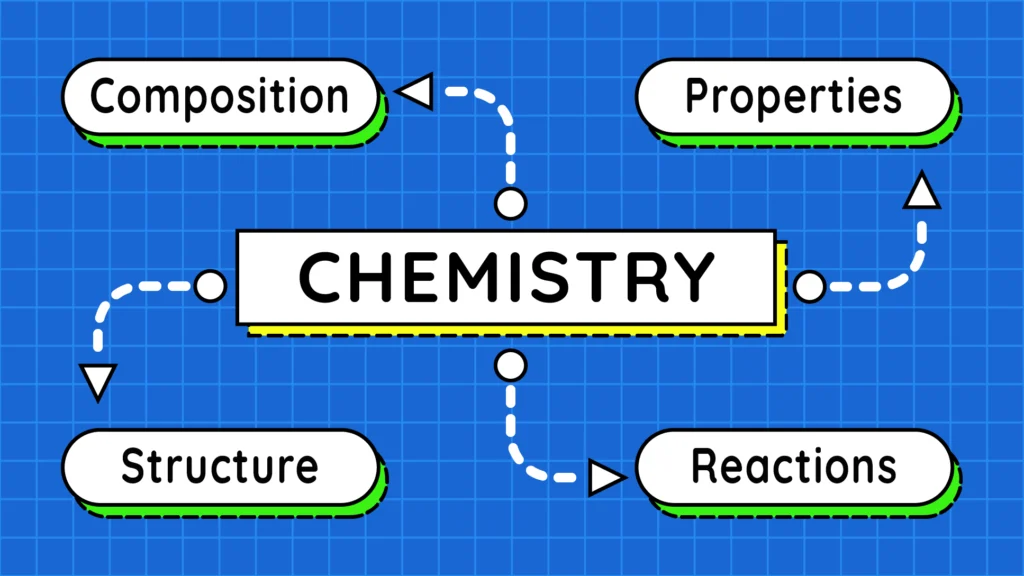
Before we begin studying chemistry, it is important to understand what this branch of science deals with. In this article, we will learn all about chemistry and the topics it covers.
But first things first – lets start with the definition of chemistry and here is one way to define it:
“Chemistry is the branch of science in which we study the composition, properties, structure and chemical reactions of matter.”
This definition tells us that chemistry primarily focuses on the following four aspects of matter:
- Composition
- Properties
- Structure
- Chemical Reactions
Let’s explore what each of these terms means to gain a deeper understanding of chemistry.
Composition of Matter:
The composition of anything includes two things: its components and the amount of those components. To understand the concept of composition, let’s make a glass of lemonade.
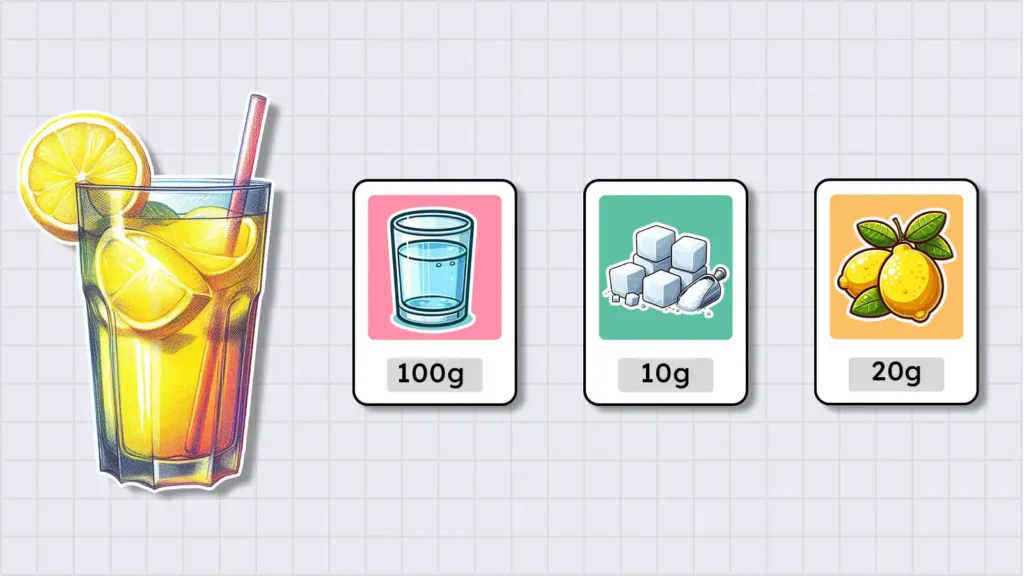
To make a glass of lemonade, we need three ingredients: water, sugar, and lemon juice. However, if you want lemonade with the exact same taste as mine, you should use 100 g of water, 10 g of sugar, and 20 g of lemon juice.
In this example, the composition of lemonade includes both the components (ingredients) and the amount of those components. The same principle applies to any other type of matter.
For some types of matter, like lemonade, composition is not particularly important. For instance, you can add a small pinch of salt, and it will still be lemonade. However, for other types of matter, such as compounds, composition is extremely important.
Let’s take a common compound as an example: water (H₂O). The composition of water includes its components—hydrogen (H) and oxygen (O)—and their exact ratio (2:1).
If we alter the composition of water in any way, we create a new kind of matter. For example:
- If we change the amount of components and add an extra oxygen atom, we get hydrogen peroxide (H2O2), which is used as a bleaching agent but is unsafe to drink.
- But if we replace the oxygen (O) with sulfur (S), we get hydrogen sulfide. Which is an extremely toxic and foul-smelling gas.
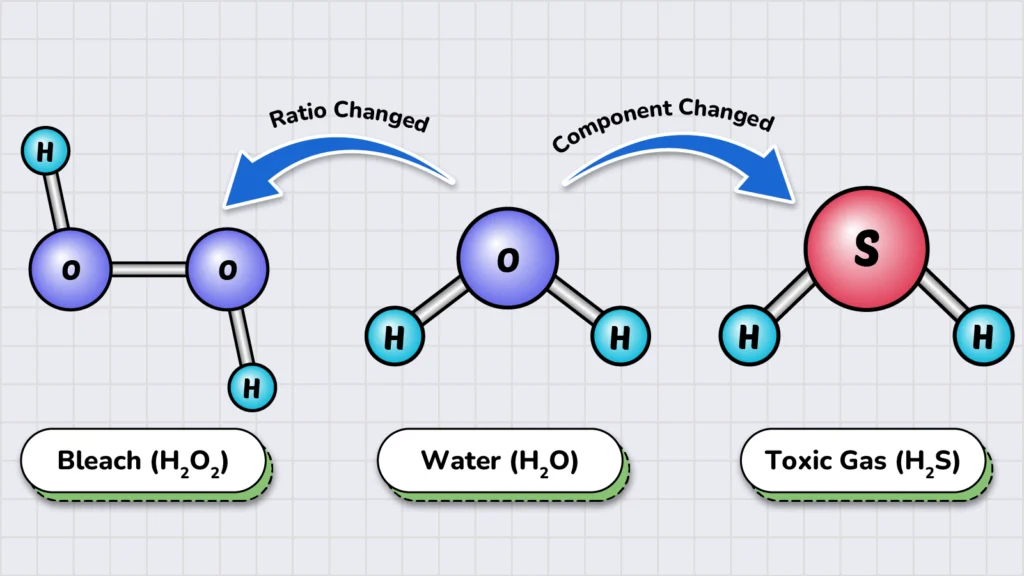
This is why studying the composition of matter is essential in chemistry—it helps us identify and distinguish different types of matter.
Properties of Matter:
The next concept we study in chemistry is the properties of matter. Properties are the characteristics of matter that are observable and measurable. They provide valuable information about a substance, such as how it reacts with other substances, whether it is flammable, or if it is toxic.
For example, both ethanol and water are colorless liquids. To distinguish between them, we need to study their properties. These properties can help us distinguish ethanol which is a toxic liquid from water.
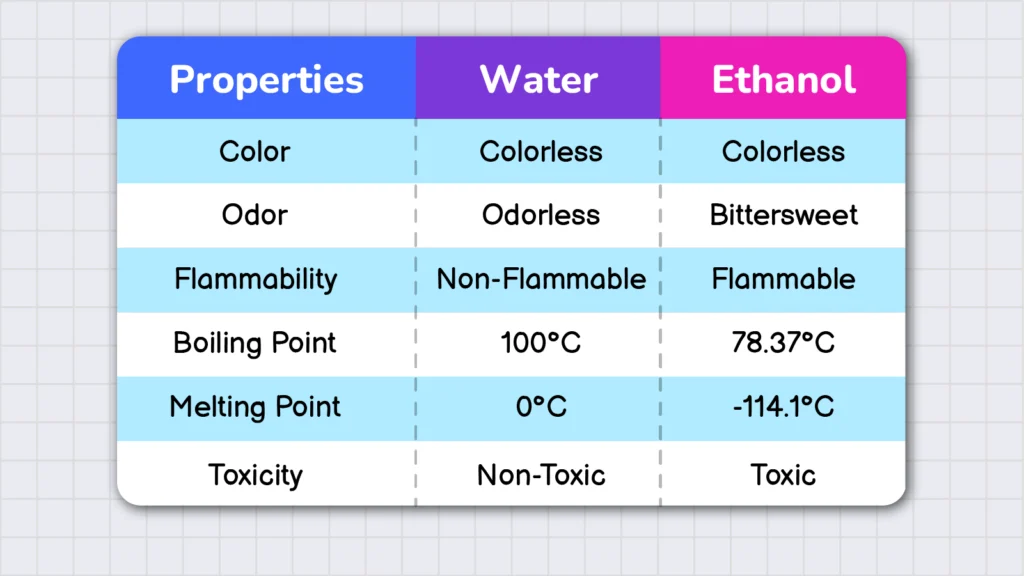
Based on how they are studied, the properties of matter are classified into two major categories: physical properties and chemical properties. Physical properties are characteristics of matter that can be observed without causing any chemical change in the substance. In contrast, chemical properties can only be observed through a chemical change.
Structure of Matter:
The third important concept we study in chemistry is the structure of matter. Structure of matter refers to the three-dimensional arrangement of its components—in other words, how atoms are arranged within a matter.
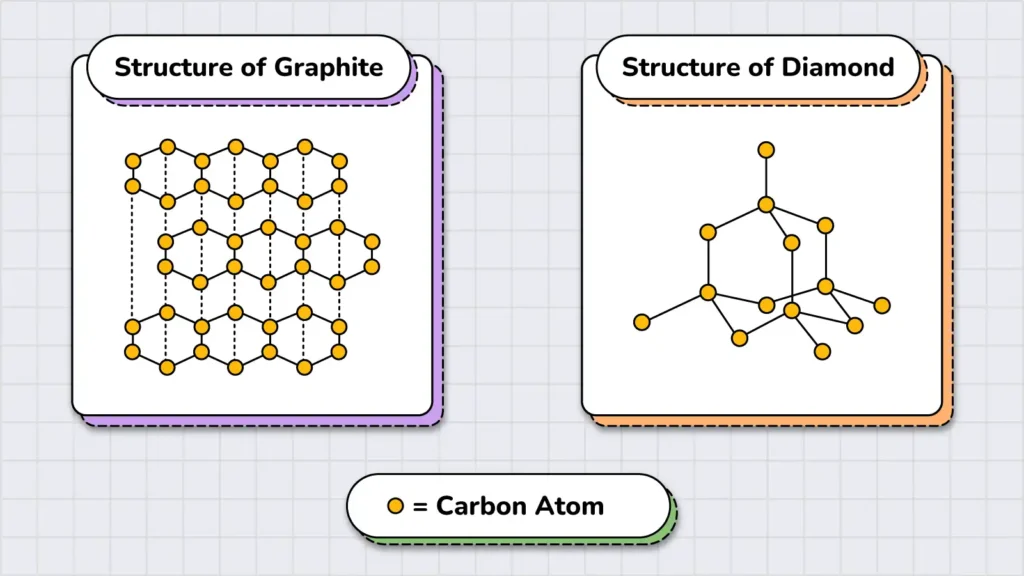
To understand the importance of structure, let’s compare a pencil and a diamond. The core of a pencil is made of graphite, and interestingly, both graphite and diamond are composed of the same element: carbon. But if they are both made of carbon, why is one so inexpensive while the other is so valuable?
The answer lies in their structure. In graphite, carbon atoms are arranged in layers, making it soft and breakable. In contrast, in diamond, carbon atoms form a strong, rigid structure, making it one of the hardest known materials. Simply changing the structure of matter gives us two completely different substances with distinct properties.
Chemical Reactions:
The final key concept we study in chemistry is chemical reactions. A chemical reaction can be defined as a change in the composition or structure of matter.
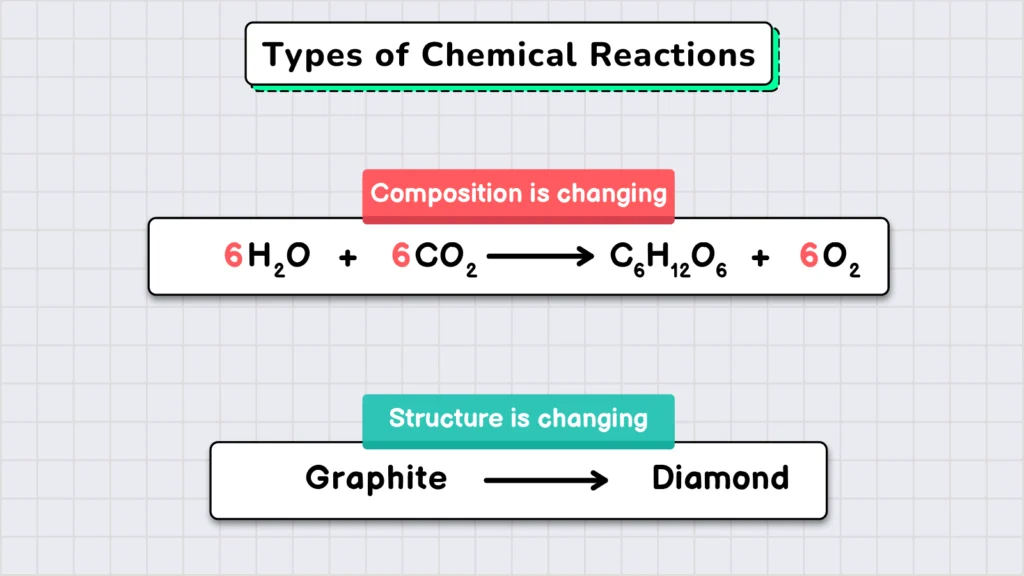
Chemical reactions happen all around us. The burning of fuel in a vehicle, the baking of delicious cookies, and the boiling of an egg – all involve some form of chemical reaction.
All of these reactions involve a change in the composition of matter. However, chemical reactions can also alter the structure of matter. For example, if we subject graphite to very high temperature and pressure, its structure changes, transforming it into a shining diamond.
Conclusion:
Therefore, if we want to understand all these aspects of matter more deeply, it’s important for us to study chemistry. It can help us gain a better understanding of ourselves and the universe around us.